Structural features of the Beta-sheet
The β-sheet is one of the two regular scaffolds of the backbone in a protein structure. The stereo-chemistry of the β-sheet was derived by Linus Pauling in 1951 (PNAS 37:729), allegedly using paper and scissors whilst he was sick in a hospital bed. This was an incredible achievement at the time as no protein structure had yet been solved.
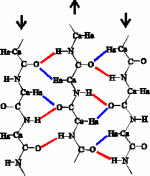
The β-sheet consists of straight segments of the protein strand held together by hydrogen bonds. In a recent study of β-sheets in high-resolution protein structures, Ho & Curmi (2002 JMB 317:291) discovered of a new feature in the interface between the strands of the β-sheet, the bifurcated hydrogen bond.
Antiparallel β-sheet Shear induced by the bifurcated hydrogen bond
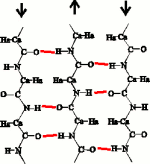
In the original Pauling antiparallel β-sheet, the C=O...H-N hydrogen bonds that hold the strands together are exactly perpendicular to the strands. This aligns residues that are cross-strand neighbours in the β-sheet.
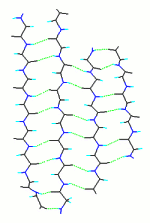
However, a number of researchers (Fraser & McRae, 1973, Conformations in Fibrous Proteins, Acad Press; Wouters & Curmi, 1995, Proteins 22:119, MacCallum, 1995 JMB 248:361.4) found a shear in antiparallel β-sheets. Here is the shear in a β-sheet from Conocavalin A.

The shear bends the hydrogen bonds, and displaces the alignment of the strands by about 0.5 Å in the C-terminal direction. Where does this shear come from? Ho & Curmi (2002, JMB 317:291) showed that the only interaction consistent with the measured geometry of the shear is formation of the Ca=Ha...O hydrogen bond (blue) in the interface between the strands.
Yet, there is some debate as to whether the C=H...O weak hydrogen bond is just an artifact in protein structures. In small-molecule crystal structures, the cohesive nature of C=H...O hydrogen bonds was convincingly demonstrated by Taylor and Kennard (1982, JACS 104:5063). Later, Derewenda and co-workers (1995, JMB 252:248) found statistical evidence for the existence of C=H...O hydrogen bonds in proteins.

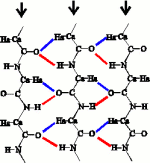
The C=O...H-N hydrogen bond and the CaHa...O hydrogen bond forms a network called the bifurcated hydrogen bond where the O atom is common to the two hydrogen bonds. In the antiparallel β-sheet, the sheet is sheared to accomodate the birfuracted hydrogen bonds. In contrast, the parallel β-sheet as described by Linus Pauling, already places the C=H atoms in contact with the O atoms. Once the bifurcated hydrogen bonds are considered, the hydrogen bonding patterns between the antiparallel (left) and parallel (right) β-sheets look very similar.
Twist of the β-sheet
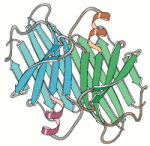
Although the original β-sheet proposed by Pauling was regular and flat, β-sheets in real proteins are normally twisted and rarely flat. This twist is biased in one direction and not the other. This twisting of the β-sheet was first described by Cyrus Chothia in 1973 [3], and an example can be seen in the diagram (of the prealbumin dimer). By convention, the observed bias in the twist is called the right-handed twist. [3] Chothia (1973) JMB 75:295.

The sense of the right-handed twist is shown in the diagram, where each strand of the β-sheet is represented by a cylinder. By placing one of the strands along the middle finger of your right hand, the natural curvature of your hand defines the sense of twisting in the plane. This bias of the right-handed twist is the same even if you flip-over the sheet or look at the β-sheet from the other side.
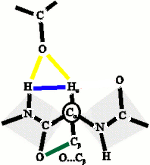
The bifurcated H-bond (yellow) constrains the protein backbone (blue). Within these constraints, the O...Cβ steric clash (green) will twist the protein backbone. Such twisted backbones will result in strands that join together to form right-hand twisted β-sheets.